
Group IA is the group of the most reactive metals.Hence, it is easy to lose electrons down the group.Nuclear attraction between the protons and valence electrons decreases.Reactivity increases down the group due to increase in electropositivity.
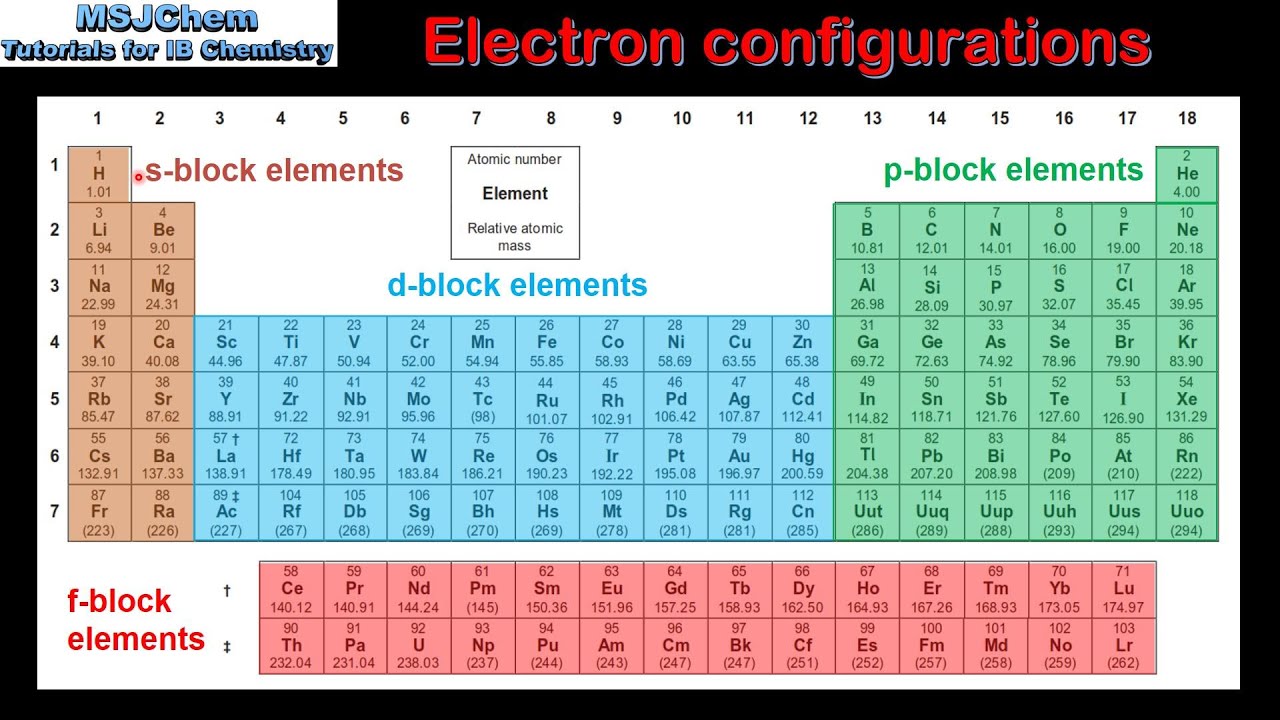
s-block elements are (IA, IIA), p-block elements are (IIIA to VIIA, 0) and metalloids are B, Si, As (Arsenic), Ge (Germanium) and Sb (Antimony).Transition or d-block elements are IB, IIB,………VIIB, VIII.Normal or Representative elements are IA, IIA, IIIA, ….VIIA, 0.15 elements from 57La (Lanthanum) to 71Lu (Lutetium) and other 15 elements from 89Ac (Actinium) to 103Lw (Lawrencium) are f-block or Inner transition elements.duplet (He) and octet state (Ne, Ar etc.) so they don’t react with other elements. They have stable electronic configuration i.e.


 0 kommentar(er)
0 kommentar(er)
